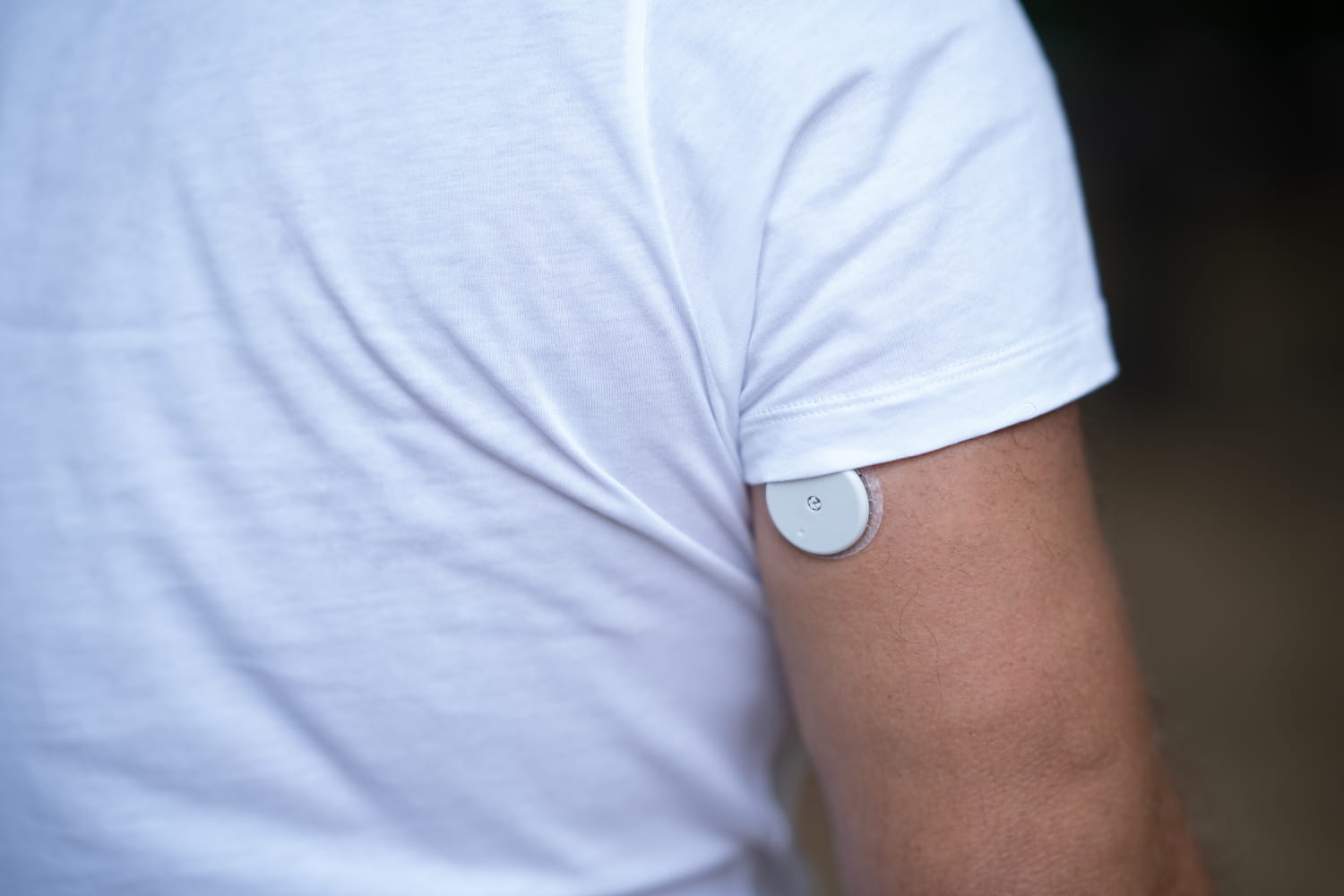
These sensors provide incorrect low glucose readings, putting patients’ lives at risk.
A global reminder. Blood glucose sensors produced by the American company Abbott are being recalled due to malfunctions that could endanger the lives of diabetic patients. Seven deaths (none in the United States) and 736 serious injuries (including 57 in the United States) have already been recorded by the manufacturer. What happened? What medical devices are affected by this recall?
Blood glucose sensors are used to measure blood sugar levels continuously, without repeated finger pricks. They stick to the skin, sense glucose levels and send the data to a phone or reader. These sensors allow people with diabetes to see if their blood sugar is rising or falling, anticipate hypo or hyperglycemia, and adjust insulin, diet or activity. They are essential for accurate, real-time disease monitoring. They have no room for error, hence the concern of health authorities since the announcement by Abbott Diabetes Care Inc. of the recall of several batches of sensors having provided incorrect measurements of low glucose levels. “If these erroneous readings go undetected over an extended period of time, they can lead to inappropriate treatment decisions in people with diabetes, such as consuming too many carbohydrates or omitting or delaying insulin injections. These decisions can result in serious health risks, including injury or death, or other less serious complications.” warn the French (ANSM) and American (FDA) drug agencies.
The sensors concerned are the FreeStyle Libre 3 and FreeStyle Libre 3 Plus models, used either with the FreeStyle libre 3 application (on smartphone) or with the mylife CamAPS FX application. FreeStyle Libre systems (all versions — not just Libre 3) are used by approximately 7 million people worldwide. Three million potentially defective sensors have been identified in the United States. No figures were given for France. Patients using such sensors have typically received notification asking them to visit FreeStyleCheck.com to see if their devices are recalled. The serial number can be found on the box or on the application.
If you are wearing a recalled sensor, “stop using it immediately.” and throw it away. You can request another sensor for free from the manufacturer’s website and use a blood glucose meter while you wait for the new sensor or to check the readings if they don’t match your usual symptoms or numbers. Abbott clarified that the malfunction does not affect the FreeStyle Libre 3 reader or application or any other FreeStyle Libre product (FreeStyle Libre Select, FreeStyle Libre 2 and FreeStyle Libre 2 Plus). For any questions, reports of adverse reactions or quality malfunctions related to the use of FreeStyle Libre 3/FreeStyle Libre 3 Plus sensors, Abbott customer service can be reached at 0800.10.11.56 or 01.45.60.34.34 Monday to Saturday from 8 a.m. to 7 p.m.
Blood glucose sensors have been around for about 20 years. The first was marketed in the United States in 1999 by Medtronic. It then arrived on the European market in 2000-2001. The first sensors were used in a medical environment for a few days, then analyzed by the doctor. They were not yet intended for continuous use by patients as today. Manufacturers Abbott and Dexcom arrived in this market in the 2010s. These are the three predominant brands of blood glucose sensors in the world, including in France.