He is especially taken by people over 60 years of age.
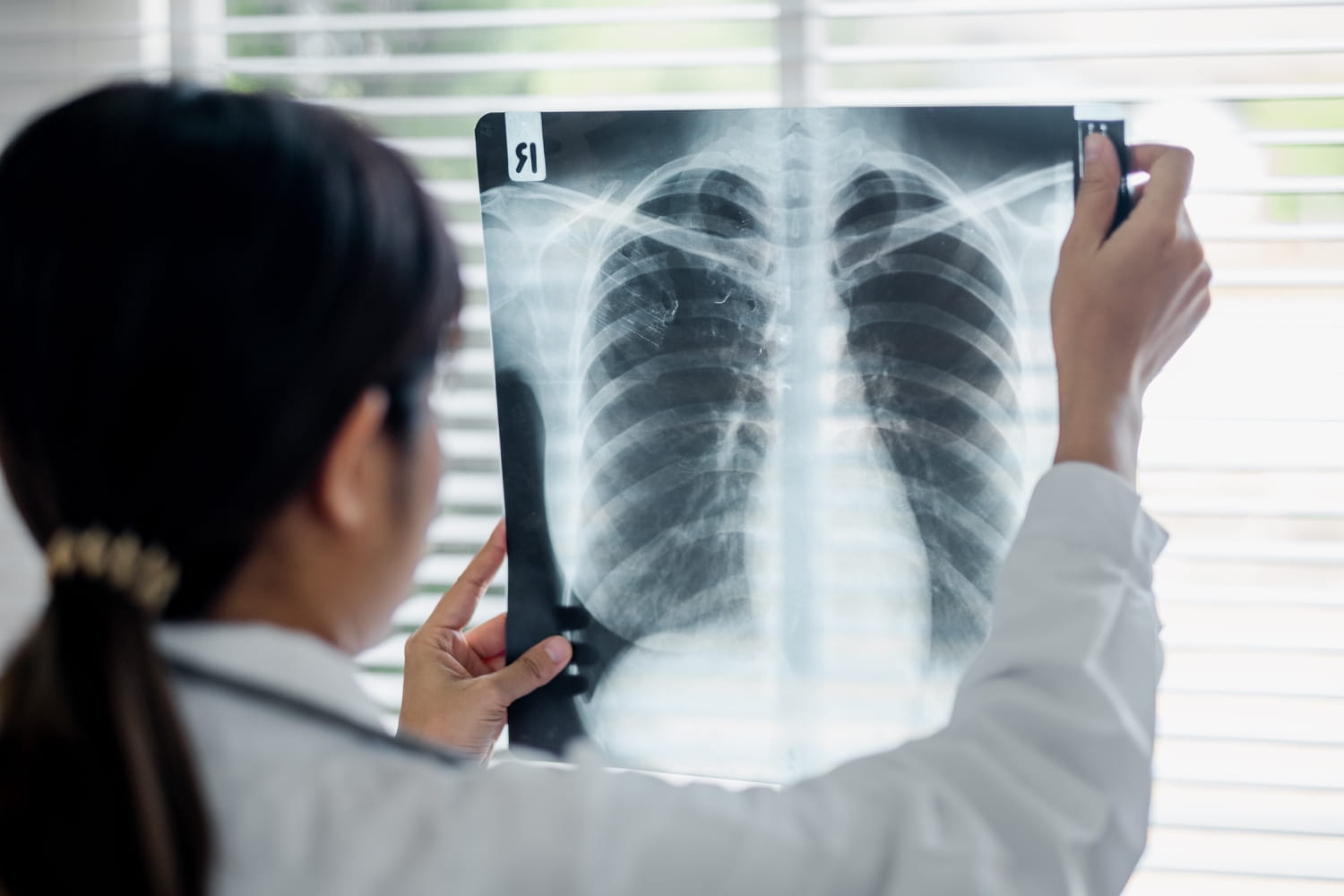
An antidepressant is the subject of a recall in French pharmacies, announces the National Medicines Safety Agency (ANSM). This withdrawal will lead to total unavailability of treatment and follows the presence of impurity at rates above European standards. Currently, 5,000 patients, mostly people over the age of 60, are treated by this drug in France.
At the origin of this recall, confirmation by the AMDIPHARM laboratory, which markets the drug, that the levels of nitrosamines present in the composition “are superior to the standards set by the European authorities”reports the ANSM. N-nitrosamines are impurities classified as probable carcinogens for humans according to EDOM (European Directorate for the Quality of Medicines and Health Care). This means that long -term exposure can potentially increase the risk of developing cancer. According to EDOM, these impurities can appear accidentally at different stages of manufacturing or when preserving the drug. However, the ANSM stresses that the production and distribution of the drug had already been suspended since the end of March 2025, pending confirmation of contamination.
The treatment concerned is Ludiomil © (Maprotiline), a drug used for patients with depression. The following lots are targeted by this recall, distributed until March 31, 2025: Ludiomil © 25 mg: lot F0016 (expiration on 05/2026) and lot F0017 (expiration on 10/2027); Ludiomil © 75 mg: lot F0018 (expiration on 03/2026). Faced with this reminder, the ANSM communicates a clear instruction: especially not to interrupt treatment alone. Brutal withdrawal exposes to significant undesirable effects (nausea, anxiety, sleep disorders, headache …). The risk linked to this brutal judgment is much more immediate and concrete than that linked to impurity.
For patients under treatment, “A new prescription from your doctor will be necessary”assures the ANSM. Alternatives exist, such as Laroxyl © or Norset ©. Patients are therefore recommended to consult their doctor “Without delay the date of the next order renewal in order to ensure continuity of treatment”. The delivery of Ludiomil © is not expected before the “End of year 2026”concludes the agency.